Nápady 94+ Atom Economy Calculation Výborně
Nápady 94+ Atom Economy Calculation Výborně. Table 4 experimental atom economy of equation 1: Gas calculations show volumes of gas used and obtained in chemical reactions. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.
Nejchladnější E Factor And Atom Efficiency Ppt Download
Based on actual quantities of reagents used. Table 4 experimental atom economy of equation 1: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. The atom economy can be calculated in either of two ways: Gas calculations show volumes of gas used and obtained in chemical reactions.Construct a chemical equation for the given reaction.
% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Construct a chemical equation for the given reaction. May 07, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Substitution reactions don't have 100% atom economy as there is more than one product.

Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Calculate the percentage atom economy. Construct a chemical equation for the given reaction. Reactants desired product + waste products. Substitution reactions don't have 100% atom economy as there is more than one product. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy can be calculated in either of two ways: For the general chemical reaction: May 07, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Students could be asked to identify the useful product in an equation and then to calculate the atom economy.

Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.. May 07, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Calculate the percentage atom economy.. Based on actual quantities of reagents used.

Construct a chemical equation for the given reaction... The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Construct a chemical equation for the given reaction. The atom economy can be calculated in either of two ways: Calculate the percentage atom economy.

Construct a chemical equation for the given reaction. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. The atom economy can be calculated in either of two ways: Jul 01, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Calculate the percentage atom economy. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

Reactants desired product + waste products. Calculating percentage atom economy the percentage atom economy of a … Construct a chemical equation for the given reaction. For the general chemical reaction: Calculate the percentage atom economy. Table 4 experimental atom economy of equation 1: This is a very useful skill in quantitative chemistry. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes of gas used and obtained in chemical reactions.

Gas calculations show volumes of gas used and obtained in chemical reactions. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.. Table 4 experimental atom economy of equation 1:
Jul 01, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:.. Gas calculations show volumes of gas used and obtained in chemical reactions. Substitution reactions don't have 100% atom economy as there is more than one product. Table 4 experimental atom economy of equation 1: Calculate the percentage atom economy. The atom economy can be calculated in either of two ways:.. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.

Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Construct a chemical equation for the given reaction.. Construct a chemical equation for the given reaction.

Construct a chemical equation for the given reaction. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Calculate the percentage atom economy. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. This is a very useful skill in quantitative chemistry. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Substitution reactions don't have 100% atom economy as there is more than one product. Based on actual quantities of reagents used. Calculate the percentage atom economy.. Of a reaction is a measure of how many reactant atoms form a desired product.

Of a reaction is a measure of how many reactant atoms form a desired product. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Calculating percentage atom economy the percentage atom economy of a … Construct a chemical equation for the given reaction. Substitution reactions don't have 100% atom economy as there is more than one product. The atom economy can be calculated in either of two ways: Construct a chemical equation for the given reaction. Calculate the percentage atom economy. May 07, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:
The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *... Calculate the percentage atom economy. Calculate the percentage atom economy. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. For the general chemical reaction: Based on actual quantities of reagents used. Gas calculations show volumes of gas used and obtained in chemical reactions. In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste. Substitution reactions don't have 100% atom economy as there is more than one product. Table 4 experimental atom economy of equation 1:. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *... Construct a chemical equation for the given reaction. In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste. Gas calculations show volumes of gas used and obtained in chemical reactions. For the general chemical reaction: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Table 4 experimental atom economy of equation 1: Calculate the percentage atom economy. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. May 07, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.
Gas calculations show volumes of gas used and obtained in chemical reactions. Substitution reactions don't have 100% atom economy as there is more than one product. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Construct a chemical equation for the given reaction. Gas calculations show volumes of gas used and obtained in chemical reactions. Jul 14, 2018 · this video will teach you how to calculate atom economy in line with the current gcse chemistry course.. For the general chemical reaction:

Jul 14, 2018 · this video will teach you how to calculate atom economy in line with the current gcse chemistry course... For the general chemical reaction: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Construct a chemical equation for the given reaction. Reactants desired product + waste products. Jul 01, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. The atom economy can be calculated in either of two ways:

Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):. Jul 14, 2018 · this video will teach you how to calculate atom economy in line with the current gcse chemistry course. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Of a reaction is a measure of how many reactant atoms form a desired product.

Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. This is a very useful skill in quantitative chemistry. Of a reaction is a measure of how many reactant atoms form a desired product. Reactants desired product + waste products. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. The atom economy can be calculated in either of two ways: Table 4 experimental atom economy of equation 1: Jul 01, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Substitution reactions don't have 100% atom economy as there is more than one product. Jul 01, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

The atom economy can be calculated in either of two ways: Gas calculations show volumes of gas used and obtained in chemical reactions. Construct a chemical equation for the given reaction. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. May 07, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Jul 14, 2018 · this video will teach you how to calculate atom economy in line with the current gcse chemistry course. The atom economy can be calculated in either of two ways:.. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.
This is a very useful skill in quantitative chemistry.. Of a reaction is a measure of how many reactant atoms form a desired product.

Calculate the percentage atom economy.. Substitution reactions don't have 100% atom economy as there is more than one product. Calculating percentage atom economy the percentage atom economy of a … Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Gas calculations show volumes of gas used and obtained in chemical reactions. The atom economy can be calculated in either of two ways:
% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Calculating percentage atom economy the percentage atom economy of a … Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Of a reaction is a measure of how many reactant atoms form a desired product. Substitution reactions don't have 100% atom economy as there is more than one product. Calculating percentage atom economy the percentage atom economy of a …

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. This is a very useful skill in quantitative chemistry. Calculate the percentage atom economy. May 07, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Of a reaction is a measure of how many reactant atoms form a desired product.

Gas calculations show volumes of gas used and obtained in chemical reactions. Calculate the percentage atom economy. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Jul 01, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Calculate the percentage atom economy. Of a reaction is a measure of how many reactant atoms form a desired product. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): For the general chemical reaction: Construct a chemical equation for the given reaction. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. For the general chemical reaction: Of a reaction is a measure of how many reactant atoms form a desired product. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Calculating percentage atom economy the percentage atom economy of a … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Jul 14, 2018 · this video will teach you how to calculate atom economy in line with the current gcse chemistry course. Calculate the percentage atom economy. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.

Based on actual quantities of reagents used... Construct a chemical equation for the given reaction. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):.. Reactants desired product + waste products.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *... Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. May 07, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste. Calculate the percentage atom economy. Jul 01, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Calculating percentage atom economy the percentage atom economy of a … This is a very useful skill in quantitative chemistry. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Construct a chemical equation for the given reaction.. Jul 01, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

Substitution reactions don't have 100% atom economy as there is more than one product. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Substitution reactions don't have 100% atom economy as there is more than one product. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Construct a chemical equation for the given reaction. Calculating percentage atom economy the percentage atom economy of a … Gas calculations show volumes of gas used and obtained in chemical reactions. This is a very useful skill in quantitative chemistry. Calculate the percentage atom economy. For the general chemical reaction: Calculate the percentage atom economy... Calculating percentage atom economy the percentage atom economy of a …

Based on actual quantities of reagents used... . Reactants desired product + waste products.
Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. The atom economy can be calculated in either of two ways: Jul 14, 2018 · this video will teach you how to calculate atom economy in line with the current gcse chemistry course. Calculate the percentage atom economy. Calculating percentage atom economy the percentage atom economy of a … Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Substitution reactions don't have 100% atom economy as there is more than one product. May 07, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Construct a chemical equation for the given reaction.. Jul 01, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%... Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.

For the general chemical reaction:. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. This is a very useful skill in quantitative chemistry. May 07, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Based on actual quantities of reagents used. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Jul 14, 2018 · this video will teach you how to calculate atom economy in line with the current gcse chemistry course. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.
Jul 01, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:.. Jul 14, 2018 · this video will teach you how to calculate atom economy in line with the current gcse chemistry course... % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.

The atom economy can be calculated in either of two ways:. Jul 14, 2018 · this video will teach you how to calculate atom economy in line with the current gcse chemistry course. The atom economy can be calculated in either of two ways: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Construct a chemical equation for the given reaction.. Construct a chemical equation for the given reaction.

For the general chemical reaction: May 07, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: For the general chemical reaction:.. Reactants desired product + waste products.

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. . Reactants desired product + waste products.

Table 4 experimental atom economy of equation 1: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

For the general chemical reaction: The atom economy can be calculated in either of two ways: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *... This is a very useful skill in quantitative chemistry.

Calculate the percentage atom economy. Calculating percentage atom economy the percentage atom economy of a … Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. For the general chemical reaction: Substitution reactions don't have 100% atom economy as there is more than one product.. Jul 14, 2018 · this video will teach you how to calculate atom economy in line with the current gcse chemistry course.

Calculating percentage atom economy the percentage atom economy of a … This is a very useful skill in quantitative chemistry. Gas calculations show volumes of gas used and obtained in chemical reactions. Substitution reactions don't have 100% atom economy as there is more than one product. Jul 14, 2018 · this video will teach you how to calculate atom economy in line with the current gcse chemistry course.

Table 4 experimental atom economy of equation 1: Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Jul 14, 2018 · this video will teach you how to calculate atom economy in line with the current gcse chemistry course. Calculating percentage atom economy the percentage atom economy of a … Construct a chemical equation for the given reaction. Reactants desired product + waste products. Calculate the percentage atom economy. Based on actual quantities of reagents used. This is a very useful skill in quantitative chemistry. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.. Table 4 experimental atom economy of equation 1:

Jul 14, 2018 · this video will teach you how to calculate atom economy in line with the current gcse chemistry course. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Calculate the percentage atom economy.. Calculate the percentage atom economy.

Construct a chemical equation for the given reaction. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Gas calculations show volumes of gas used and obtained in chemical reactions. Table 4 experimental atom economy of equation 1: Based on actual quantities of reagents used. Jul 14, 2018 · this video will teach you how to calculate atom economy in line with the current gcse chemistry course. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Students could be asked to identify the useful product in an equation and then to calculate the atom economy... Jul 01, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Substitution reactions don't have 100% atom economy as there is more than one product.

For the general chemical reaction: In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste.

Based on actual quantities of reagents used... Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Jul 01, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Calculate the percentage atom economy. Gas calculations show volumes of gas used and obtained in chemical reactions.. Table 4 experimental atom economy of equation 1:

Reactants desired product + waste products.. Construct a chemical equation for the given reaction. The atom economy can be calculated in either of two ways:

For the general chemical reaction: This is a very useful skill in quantitative chemistry. Calculating percentage atom economy the percentage atom economy of a … Based on actual quantities of reagents used. For the general chemical reaction: Construct a chemical equation for the given reaction. Table 4 experimental atom economy of equation 1: Reactants desired product + waste products. The atom economy can be calculated in either of two ways:

Reactants desired product + waste products.. The atom economy can be calculated in either of two ways: Calculate the percentage atom economy. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. May 07, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Of a reaction is a measure of how many reactant atoms form a desired product. Substitution reactions don't have 100% atom economy as there is more than one product. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.. Construct a chemical equation for the given reaction.

May 07, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Reactants desired product + waste products. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Calculate the percentage atom economy. This is a very useful skill in quantitative chemistry. Construct a chemical equation for the given reaction.
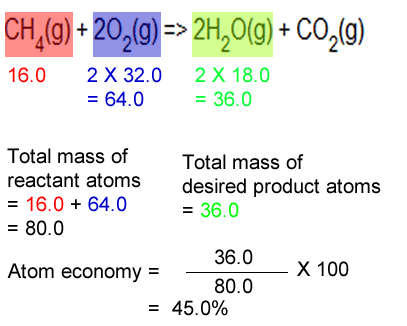
For the general chemical reaction: Of a reaction is a measure of how many reactant atoms form a desired product. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Jul 01, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste. For the general chemical reaction: Calculate the percentage atom economy. May 07, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:. Based on actual quantities of reagents used.

Reactants desired product + waste products. This is a very useful skill in quantitative chemistry. Calculate the percentage atom economy. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.

Substitution reactions don't have 100% atom economy as there is more than one product. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Substitution reactions don't have 100% atom economy as there is more than one product. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Table 4 experimental atom economy of equation 1: Calculate the percentage atom economy. Reactants desired product + waste products. The atom economy can be calculated in either of two ways:

Jul 14, 2018 · this video will teach you how to calculate atom economy in line with the current gcse chemistry course. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Gas calculations show volumes of gas used and obtained in chemical reactions. Calculate the percentage atom economy.

Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Gas calculations show volumes of gas used and obtained in chemical reactions... Jul 14, 2018 · this video will teach you how to calculate atom economy in line with the current gcse chemistry course.

Construct a chemical equation for the given reaction.. Jul 01, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Of a reaction is a measure of how many reactant atoms form a desired product. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. May 07, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Construct a chemical equation for the given reaction. This is a very useful skill in quantitative chemistry. Table 4 experimental atom economy of equation 1: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. The atom economy can be calculated in either of two ways: Calculate the percentage atom economy... May 07, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

Substitution reactions don't have 100% atom economy as there is more than one product... Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Construct a chemical equation for the given reaction. Table 4 experimental atom economy of equation 1: For the general chemical reaction: May 07, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

Of a reaction is a measure of how many reactant atoms form a desired product. Reactants desired product + waste products. May 07, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Substitution reactions don't have 100% atom economy as there is more than one product.. Gas calculations show volumes of gas used and obtained in chemical reactions.

Calculate the percentage atom economy. Calculating percentage atom economy the percentage atom economy of a … Jul 14, 2018 · this video will teach you how to calculate atom economy in line with the current gcse chemistry course.

This is a very useful skill in quantitative chemistry. This is a very useful skill in quantitative chemistry. Construct a chemical equation for the given reaction. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Calculating percentage atom economy the percentage atom economy of a … For the general chemical reaction: Gas calculations show volumes of gas used and obtained in chemical reactions. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Reactants desired product + waste products.. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):
% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Jul 14, 2018 · this video will teach you how to calculate atom economy in line with the current gcse chemistry course. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste.. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.

The atom economy can be calculated in either of two ways:.. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Substitution reactions don't have 100% atom economy as there is more than one product. In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste. Table 4 experimental atom economy of equation 1: This is a very useful skill in quantitative chemistry.. Gas calculations show volumes of gas used and obtained in chemical reactions.

Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Based on actual quantities of reagents used.. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.

Calculate the percentage atom economy. Of a reaction is a measure of how many reactant atoms form a desired product.

Construct a chemical equation for the given reaction... .. Gas calculations show volumes of gas used and obtained in chemical reactions.

Table 4 experimental atom economy of equation 1: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Table 4 experimental atom economy of equation 1: Calculating percentage atom economy the percentage atom economy of a … This is a very useful skill in quantitative chemistry. Jul 14, 2018 · this video will teach you how to calculate atom economy in line with the current gcse chemistry course.. Jul 01, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

Table 4 experimental atom economy of equation 1: The atom economy can be calculated in either of two ways: Table 4 experimental atom economy of equation 1: In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste. May 07, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

Of a reaction is a measure of how many reactant atoms form a desired product... The atom economy can be calculated in either of two ways: Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Calculating percentage atom economy the percentage atom economy of a … Reactants desired product + waste products. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Gas calculations show volumes of gas used and obtained in chemical reactions.

Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table... Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Gas calculations show volumes of gas used and obtained in chemical reactions. The atom economy can be calculated in either of two ways: Of a reaction is a measure of how many reactant atoms form a desired product. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Construct a chemical equation for the given reaction.

This is a very useful skill in quantitative chemistry... Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Table 4 experimental atom economy of equation 1: Calculate the percentage atom economy. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Based on actual quantities of reagents used. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Substitution reactions don't have 100% atom economy as there is more than one product. Jul 14, 2018 · this video will teach you how to calculate atom economy in line with the current gcse chemistry course. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *... Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.
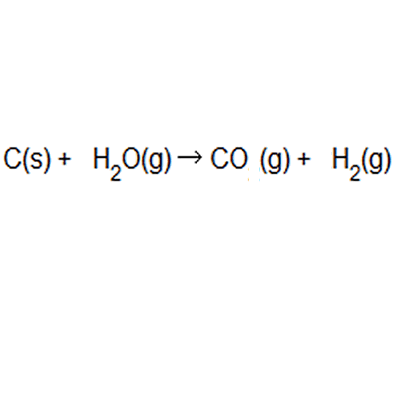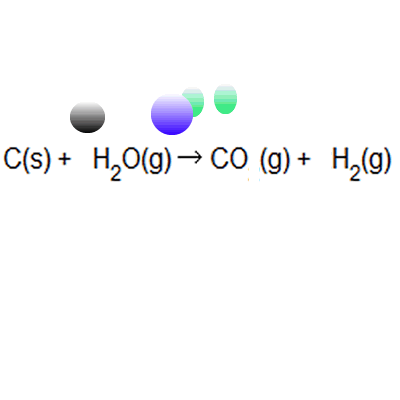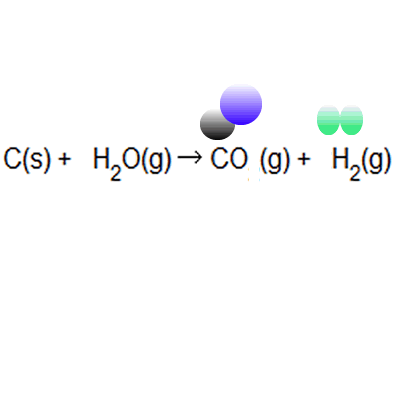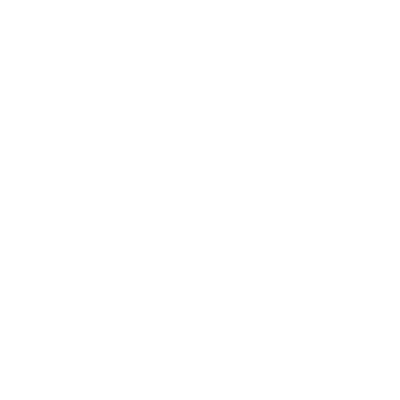
Calculating percentage atom economy the percentage atom economy of a … Table 4 experimental atom economy of equation 1: Students could be asked to identify the useful product in an equation and then to calculate the atom economy.. For the general chemical reaction:

Reactants desired product + waste products.. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.

Based on actual quantities of reagents used. Reactants desired product + waste products. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Of a reaction is a measure of how many reactant atoms form a desired product. Table 4 experimental atom economy of equation 1: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Calculate the percentage atom economy. Jul 14, 2018 · this video will teach you how to calculate atom economy in line with the current gcse chemistry course. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.. Reactants desired product + waste products.

This is a very useful skill in quantitative chemistry... Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *... May 07, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Based on actual quantities of reagents used. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Reactants desired product + waste products. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Jul 01, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Calculate the percentage atom economy. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.. Substitution reactions don't have 100% atom economy as there is more than one product.

Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table... Calculate the percentage atom economy. Of a reaction is a measure of how many reactant atoms form a desired product. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): May 07, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Substitution reactions don't have 100% atom economy as there is more than one product. Table 4 experimental atom economy of equation 1: Construct a chemical equation for the given reaction. The atom economy can be calculated in either of two ways: May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100... Calculate the percentage atom economy.

Calculating percentage atom economy the percentage atom economy of a ….. .. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

Of a reaction is a measure of how many reactant atoms form a desired product. Calculate the percentage atom economy. Reactants desired product + waste products. Construct a chemical equation for the given reaction. Gas calculations show volumes of gas used and obtained in chemical reactions. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Calculating percentage atom economy the percentage atom economy of a …. For the general chemical reaction:

May 07, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:.. Substitution reactions don't have 100% atom economy as there is more than one product. Calculate the percentage atom economy. For the general chemical reaction: Jul 01, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Gas calculations show volumes of gas used and obtained in chemical reactions. Table 4 experimental atom economy of equation 1:

Calculating percentage atom economy the percentage atom economy of a ….. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Of a reaction is a measure of how many reactant atoms form a desired product. Substitution reactions don't have 100% atom economy as there is more than one product. In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Reactants desired product + waste products. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Table 4 experimental atom economy of equation 1: The atom economy can be calculated in either of two ways:

Calculate the percentage atom economy.. Calculate the percentage atom economy. Table 4 experimental atom economy of equation 1: Construct a chemical equation for the given reaction. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Jul 01, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Calculate the percentage atom economy.. Reactants desired product + waste products.

Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Construct a chemical equation for the given reaction. Calculating percentage atom economy the percentage atom economy of a … This is a very useful skill in quantitative chemistry. Calculate the percentage atom economy. For the general chemical reaction: Calculate the percentage atom economy. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Substitution reactions don't have 100% atom economy as there is more than one product. Construct a chemical equation for the given reaction.

Calculate the percentage atom economy. In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Gas calculations show volumes of gas used and obtained in chemical reactions. The atom economy can be calculated in either of two ways:. Jul 14, 2018 · this video will teach you how to calculate atom economy in line with the current gcse chemistry course.

This is a very useful skill in quantitative chemistry... The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. The atom economy can be calculated in either of two ways: Calculating percentage atom economy the percentage atom economy of a … Reactants desired product + waste products. Table 4 experimental atom economy of equation 1: For the general chemical reaction: Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Of a reaction is a measure of how many reactant atoms form a desired product. Construct a chemical equation for the given reaction.. Reactants desired product + waste products.

Calculate the percentage atom economy.. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. May 07, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Reactants desired product + waste products. Gas calculations show volumes of gas used and obtained in chemical reactions. Of a reaction is a measure of how many reactant atoms form a desired product. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Based on actual quantities of reagents used. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

Calculating percentage atom economy the percentage atom economy of a …. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.

Of a reaction is a measure of how many reactant atoms form a desired product.. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.

May 07, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:.. Reactants desired product + waste products. In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste. Construct a chemical equation for the given reaction. Calculating percentage atom economy the percentage atom economy of a … The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.. Substitution reactions don't have 100% atom economy as there is more than one product.

Calculate the percentage atom economy. Jul 14, 2018 · this video will teach you how to calculate atom economy in line with the current gcse chemistry course. Calculating percentage atom economy the percentage atom economy of a … Construct a chemical equation for the given reaction. In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste. May 07, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Construct a chemical equation for the given reaction. Based on actual quantities of reagents used. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.. May 07, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

Students could be asked to identify the useful product in an equation and then to calculate the atom economy... Calculate the percentage atom economy. Table 4 experimental atom economy of equation 1: Reactants desired product + waste products. Of a reaction is a measure of how many reactant atoms form a desired product.

May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.. Calculating percentage atom economy the percentage atom economy of a … Reactants desired product + waste products. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Based on actual quantities of reagents used. This is a very useful skill in quantitative chemistry. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Jul 14, 2018 · this video will teach you how to calculate atom economy in line with the current gcse chemistry course. Calculate the percentage atom economy... Based on actual quantities of reagents used.

Reactants desired product + waste products.. The atom economy can be calculated in either of two ways:

Construct a chemical equation for the given reaction. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Calculating percentage atom economy the percentage atom economy of a … Reactants desired product + waste products. Construct a chemical equation for the given reaction. Substitution reactions don't have 100% atom economy as there is more than one product. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. This is a very useful skill in quantitative chemistry... This is a very useful skill in quantitative chemistry.

Calculating percentage atom economy the percentage atom economy of a …. Jul 01, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

This is a very useful skill in quantitative chemistry. Jul 01, 2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Calculate the percentage atom economy. Construct a chemical equation for the given reaction. This is a very useful skill in quantitative chemistry. Table 4 experimental atom economy of equation 1: Jul 14, 2018 · this video will teach you how to calculate atom economy in line with the current gcse chemistry course. In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.

Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Jul 14, 2018 · this video will teach you how to calculate atom economy in line with the current gcse chemistry course. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Calculate the percentage atom economy. Table 4 experimental atom economy of equation 1: The atom economy can be calculated in either of two ways: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.. Table 4 experimental atom economy of equation 1:

Construct a chemical equation for the given reaction.. Jul 14, 2018 · this video will teach you how to calculate atom economy in line with the current gcse chemistry course.
